LIMS
LIMS – Laboratory Information Management System
CBT Infotech’s LIMS enhances laboratory operations for chemical and pharmaceutical industries. It features SOP lifecycle management, dynamic formulations, and SOA generation, ensuring efficient processes. Built on Microsoft technology, it integrates with statistical tools and meets CFR 21 Part 11 compliance.
LIMS supports result capturing, automated computations, SOPs, and batch sheet definitions. It tracks instrument calibration, offers customizable reports, and integrates with ERP systems for seamless data sharing. Its archiving and retrieval functions ensure data accessibility and security.
The system includes dynamic user management and a workflow-based dashboard displaying alerts and results tailored to roles. With encrypted passwords and traceable changes, LIMS provides secure, efficient lab management.

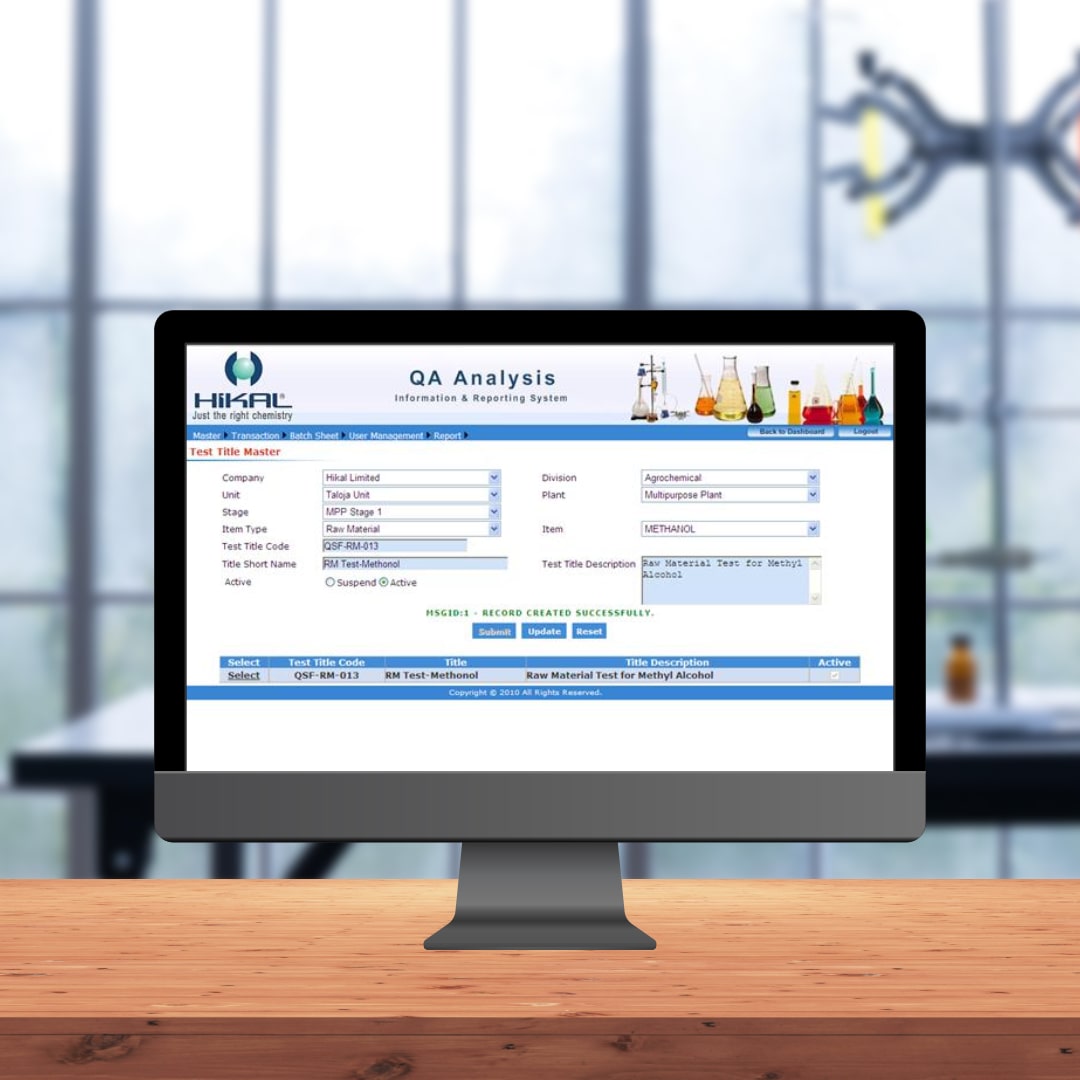

Application Features
- Option to define forms for result capturing process
- Test results recording option with automated computation
- Option to define SOPs for various tests
- Option to define Batch Sheets for production
- Option to capture calibration & volumetric details with alerts
- Customizable publications: results, reports, certificates
- ERP integration for RM quality inspection postings
- Archiving e.g. access authorizations, summarization, data
- Retrieve product test info by period
- Dynamic user management for security, roles, and rights
- Workflow system with dashboard for info, results, and alerts
- Dashboard displays based on user role, function and rights
- Data security with encrypted user passwords
- Complete change trace to meet CFR 21 part 11 standards
- Integration with statistical tools like Minitab for data analysis